Project overview
Skin wound healing re-establishes the epithelial barrier after disease or injury. During wound healing, keratinocytes surrounding the wound bed proliferate and migrate into the wound bed. Prior studies have shown that wound healing is controlled not only by biochemical signals but also by mechanical forces. In this project, we develop a cellular dynamic finite element model (CeldyFEM) to study the effects of different guidance mechanisms of cell migration and the influence of both biochemical and mechanical cues on re-epithelialization process during wound healing. Our study suggests that biochemical cues can guide keratinocyte migration with improved directionality and persistence, while mechanical cues can guide keratinocyte migration with improved coordination so neighboring keratinocyte migrate collectively and efficiently towards the wound bed. View more details of CeldyFEM in model background and our publication Zhao, et al., J. Royal Soc. Interface, 2017.
Model and Measurements
Model of the skin tissue, cell migration mechanisms under biochemical signal (chemotaxis, chemokinesis) or mechanical signal (cohesotaxis), and metrics to measure the efficiency of re-epithelialization.
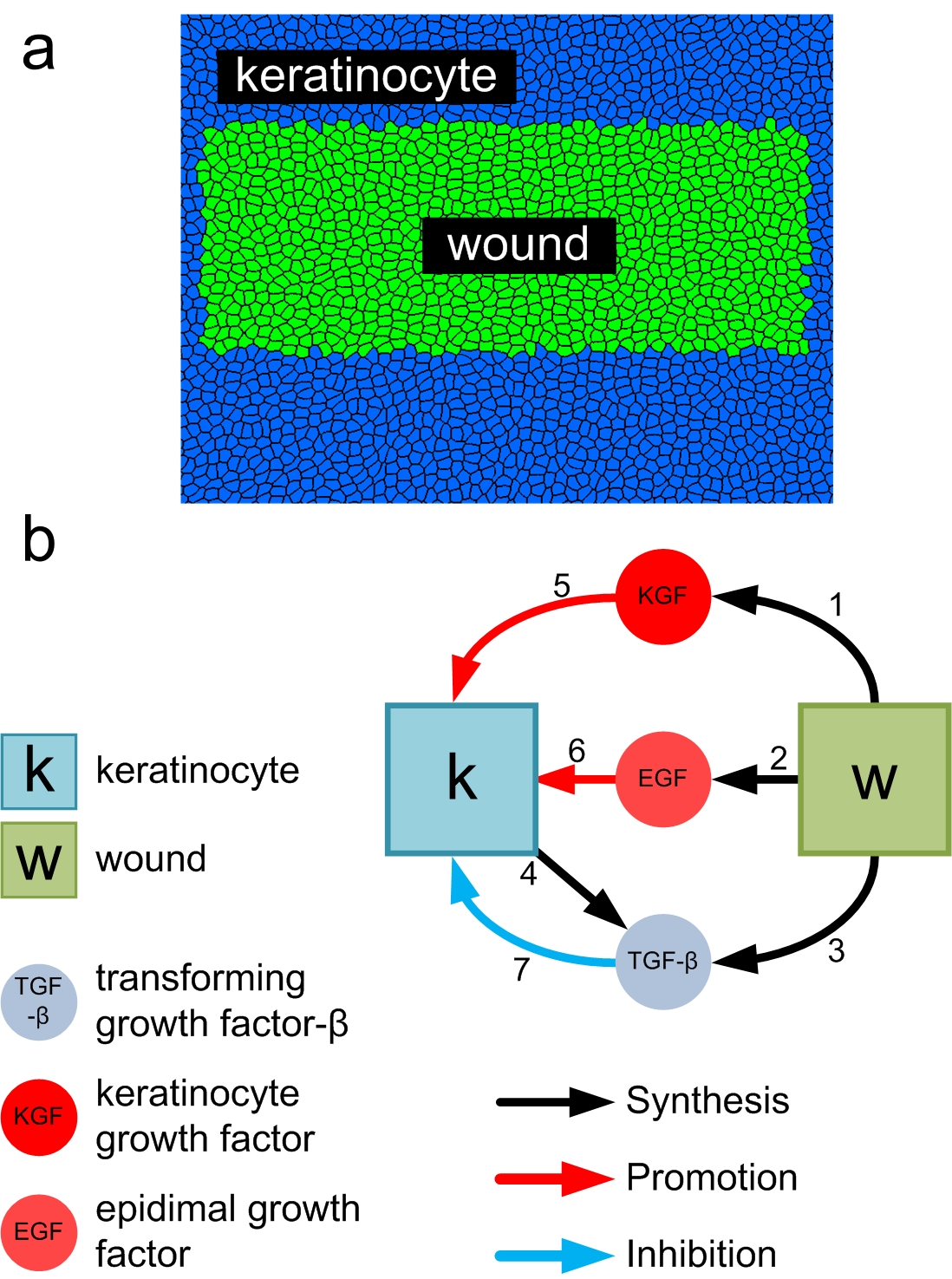
(a) Top-view of the wound tissue. (b) Intracellular network of growth factor important for skin wound healing.
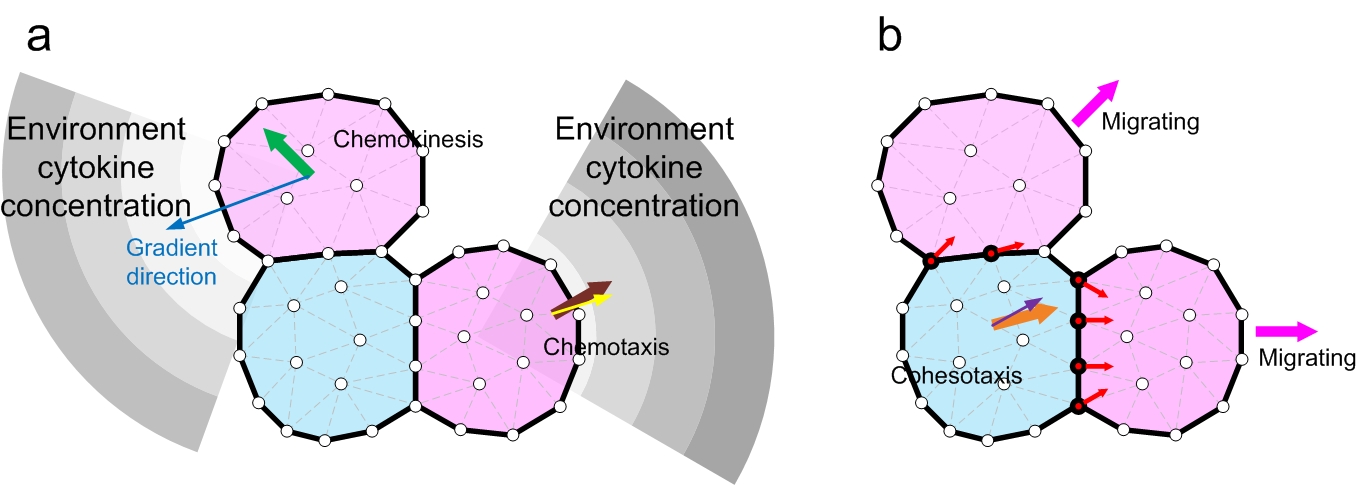
Three cell migration mechansims are compared in this project: chemotaxis, cohesotaxis and chemokinesis. (a) Chemokinesis: EGF gradient (blue arrow) activates cell migration but cell move in random direction (green arrow). Chemotaxis: migration direction is taken as the largest growth factor gradient (brown arrow), plus a random deviating angle (yellow arrow). (b) Cohesotaxis: cell migrates along the direction of the vectors of local intercellular contraction force. The contraction force vectors (red arrows) on each vertex (red vertices) of the cell boundary with a neighboring cell are summed, and the overall vector (orange arrow), plus a random deviating angle (purple) gives the migration direction.
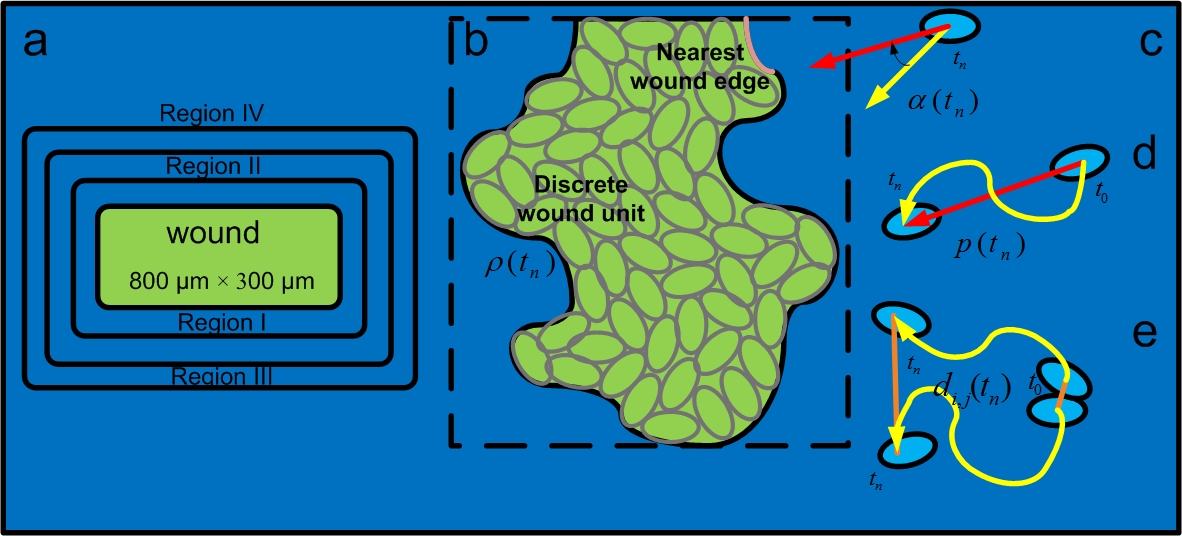
Wound geometry and quantifying re-epithelialization efficiency. (a) The size of the wound is 800 μm × 300 μm. (b) Wound closure ratio: the number of remaining discrete wound element units in the wound bed by the total number of discrete wound element units before re-epithelialization. (c) Migration direction angle: angle between the direction of migration (yellow arrow) and the direction of the cell to the nearest wound edge (red arrow). (d) Migration persistence: the ratio of the distance from the current position of the cell to its original position (red line segment), divided by the length of the traversed path (yellow curve). (e) Normalized pair separation distance: the separation distance between a pair of cells which were initially neighbors (orange line), normalized by the average length of cell traversed path (yellow curve).
Results
Re-epithelialization efficiency under different mechanisms
Re-epithelialization under cohesotaxis mechanism. Blue: keratinocyte; Light blue: migrating keratinocyte; Green: wound element.
Re-epithelialization under cohesotaxis mechanism. Blue: keratinocyte; Light blue: migrating keratinocyte; Green: wound element.
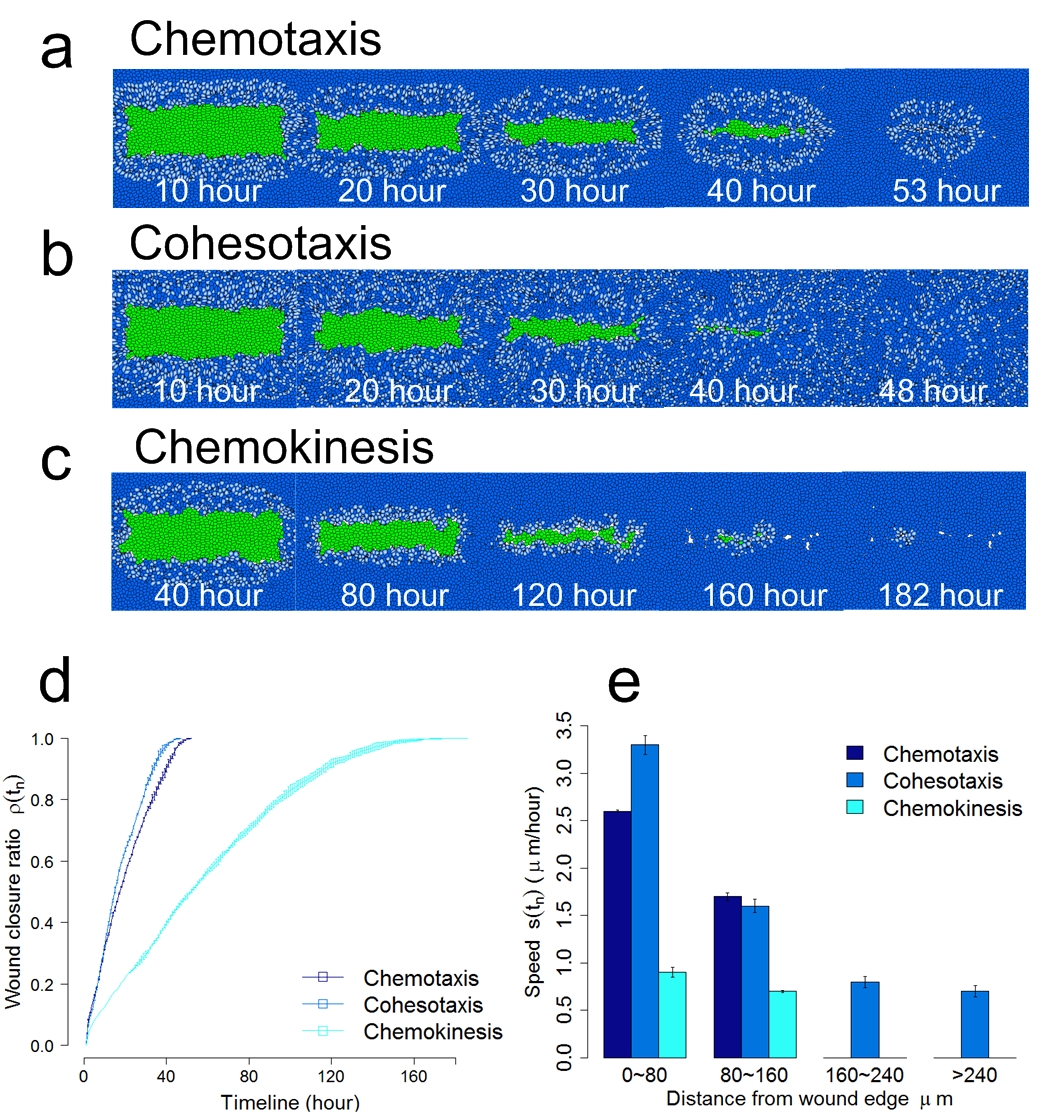
(a-c) Tissue snapshots under different guidance mechanisms of cell migration. (d) Wound closure ratio over time under the three different guidance mechanisms. (e) Average migration speed of migrating keratinocytes during re-epithelialization.
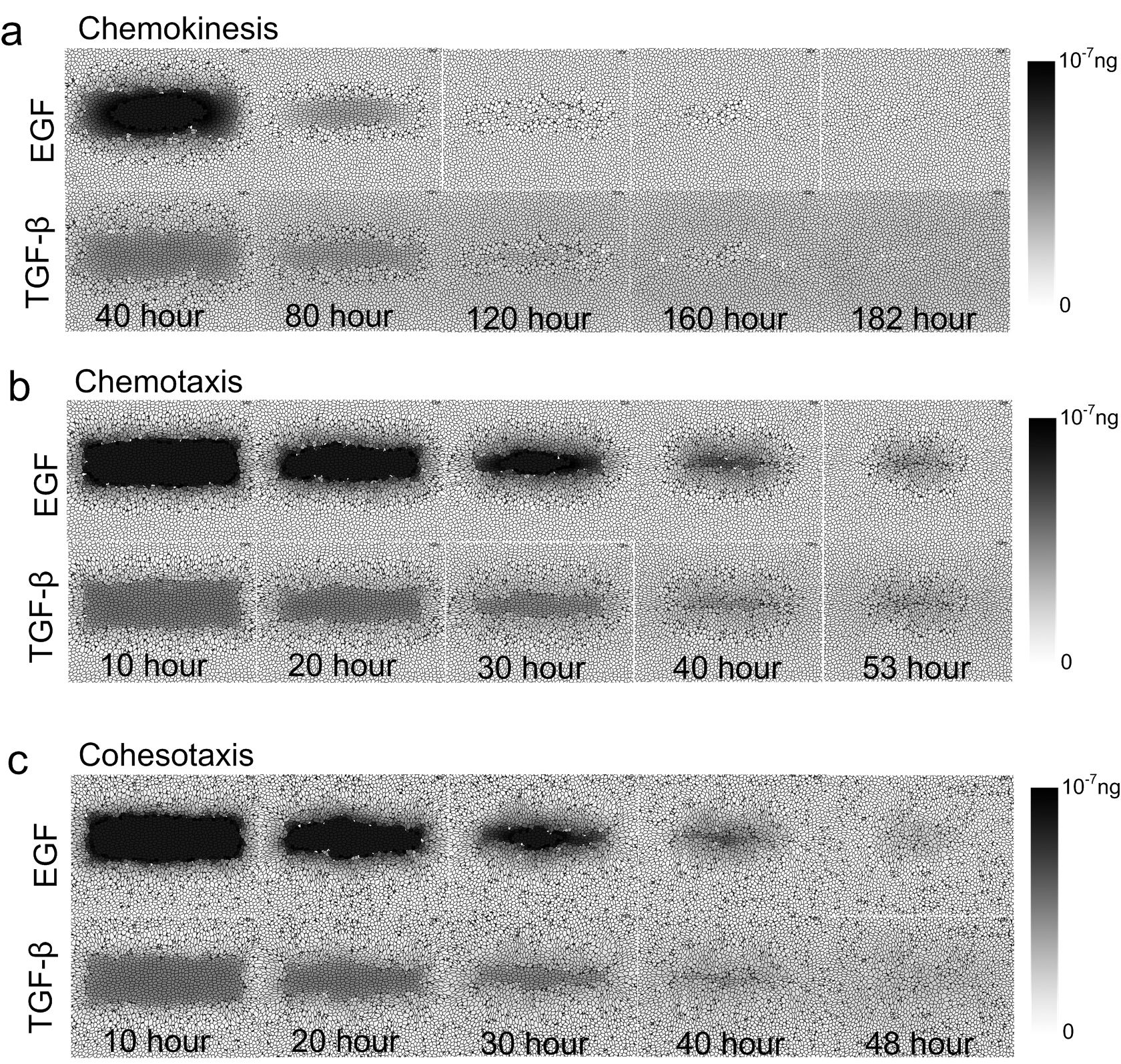
Concentration of growth factors under three different guidance mechanisms of cell migration.
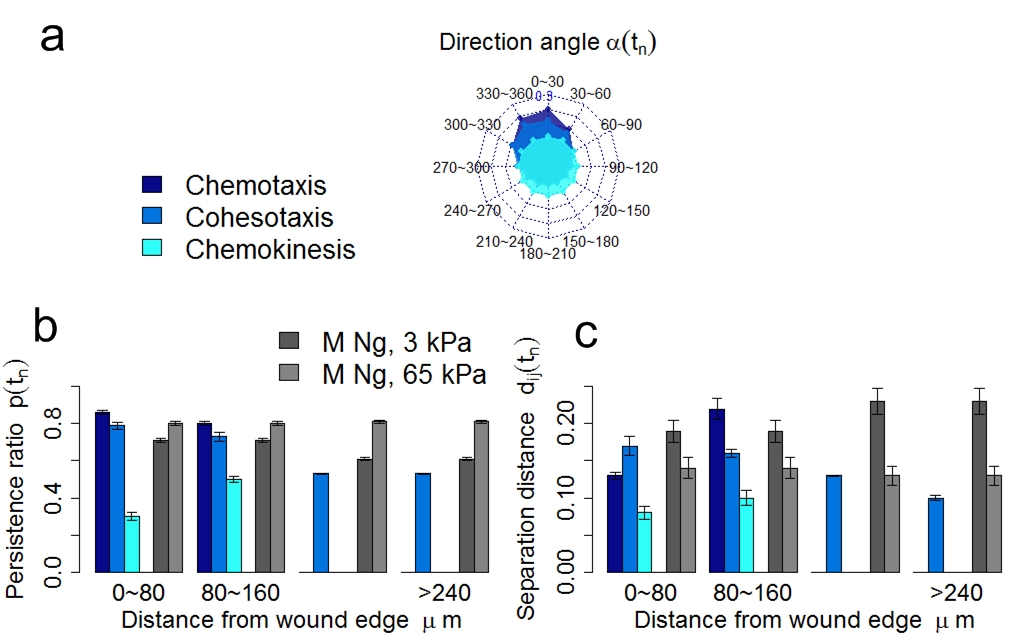
(a) Radar chart of the distribution of direction angle of migrating keratinocyte accumulated during the full process of re-epithelialization. The complete angle range 0 ~ 360 is divided into 12 intervals. Each spoke of the radar chart shows the proportion of direction angle within that specific interval. (b-c) The persistence ratio and the normalized cell separation distance of migrating keratinocytes under the three guidance mechanisms at varying distances from the wound edge during re-epithelialization. Data of migration persistence and normalized separation distance from in vitro study using matrix of different stiffness (3 kPa and 65 kPa) are also shown for comparison (Ng, et al., J. Cell Biol., 2012).
Results of re-epithelialization efficiency under different cell migration mechanisms showed that although biochemical cues act on the skin tissue locally, they can direct the migration of keratinocytes with higher persistence towards the wound bed. In contrast, mechanical cues affect the skin tissue globally and can better coordinate the collective migration of keratinocytes through local cell-cell contacts. Furthermore, the details of cell shape plays important role in guiding collective cell migration for in-time re-epithelialization.
Spatio-temporal patterns of cell proliferation under chemotaxis and cohesotaxis
Proliferation provides fresh new cells for wound repair. We study how different patterns of cell proliferation of a population of cells may arise from mechanical and biochemical guidance cues of cell migration and how these patterns of cell proliferation under chemotaxis and cohesotaxis affect the process of re-epithelialization. We divided the whole tissue of size 1600 micrometer times 700 micrometers into 56 blocks. Blocks directly covering the wound bed belong to the central region; blocks immediately neighboring the wound bed belong to the surrounding region. The time-course of re-epithelialization is divided into four intervals, 0 ~ 12 hours, 13 ~ 24 hours, 25 ~ 36 hours, 37 ~ 48 hours. The proliferation index of keratinocyte in one region at one time interval is then calculated as ratio of the number of keratinocytes that are newly generated inside that region during that time interval over the average number of keratinocytes inside that region during that time interval.
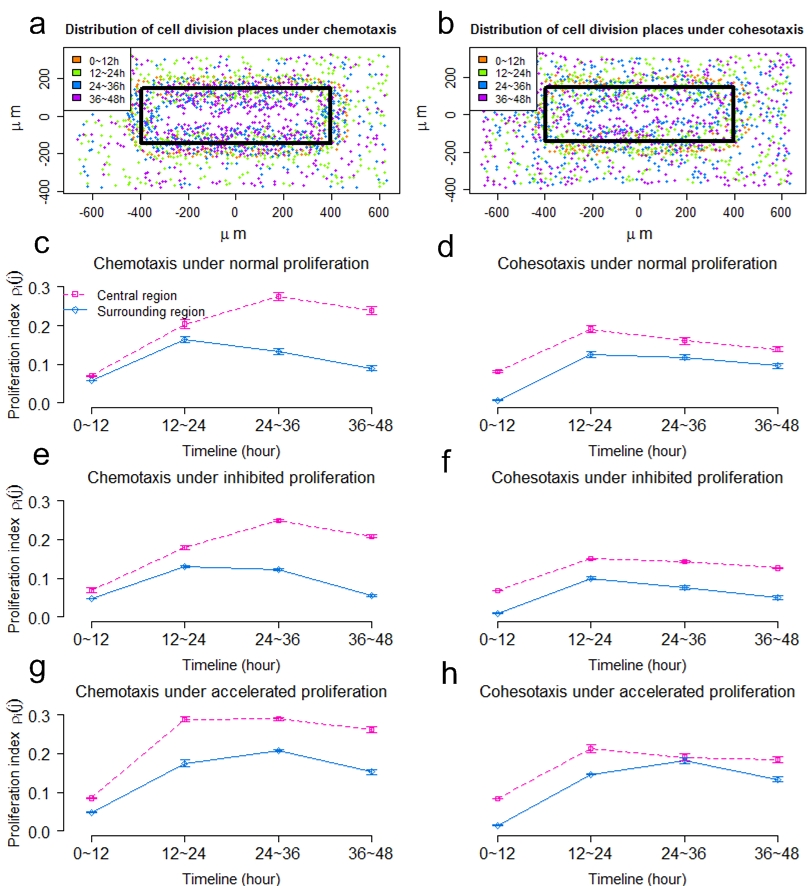
(a-b) Distributions of dividing keratinocytes under chemotaxis and cohesotaxis. Dividing cells are colored by division time. (c, e, g) The proliferation index of the central region and the surrounding region over time under chemotaxis, with normal, inhibited and accelerated keratinocyte proliferation, respectively. (d, f, h) The keratinocyte proliferation index of the central region and the surrounding region over time under cohesotaxis, with normal, inhibited and accelerated keratinocyte proliferation, respectively.
The spatial proliferating pattern under chemotaxis is more consistent with that of a previous experimental study compared to that under cohesotaxis, which showed that there exists a burst of cell proliferation at the wound margin during the early stage of re-epithelialization (Garlick, et al. Tissue En., 2007). The temporal pattern of keratinocyte proliferation under chemotaxis is consistent with the observation in recent experimental study, in which it was found that the epidermal proliferation index in the wound region remained at a high level, while proliferation in the surrounding region decreased significantly (Safferling, et al. J. Cell Biol., 2013). These similarities suggest that biochemical cues may play dominant roles in guiding migration of cells located in the wound surrounding region, while it plays negligible roles for cells located in regions distant from the wound bed.
Disturbed cell-cell adhesion under cohesotaxis
Cell-cell adhesions are critical in coordinating collective cell migration in both in vivo and in vitro studies. Studies provided evidence that mechanical tension are transmitted through E-cadherin from neighboring cells when responding to external mechanical stimulus. Many factors are known to be regulators of cell-cell adhesions. The inhibition of myosin-II rapidly decreases cadherin-based cell adhesiveness (Shewan, et al., Am. Soc. Cell Biol., 2005) and the depletion of αE-catenin significantly affects the transmission of mechanical force through cell-cell contacts (Borghi, et al., PNAS., 2012). We explored how disruption of cell-cell adhesion affect the re-epithelialization efficiency under the cohesotaxis where cells migrate following the contraction signal transmitted from neighboring cells. We first studied the effect of inhibition of myosin-II activity by decreasing the adhesion due to cadherin between keratinocytes. We then reduced the levels of αE-catenin depletion by gradually inhibiting the contraction signal transmission between keratinocytes.
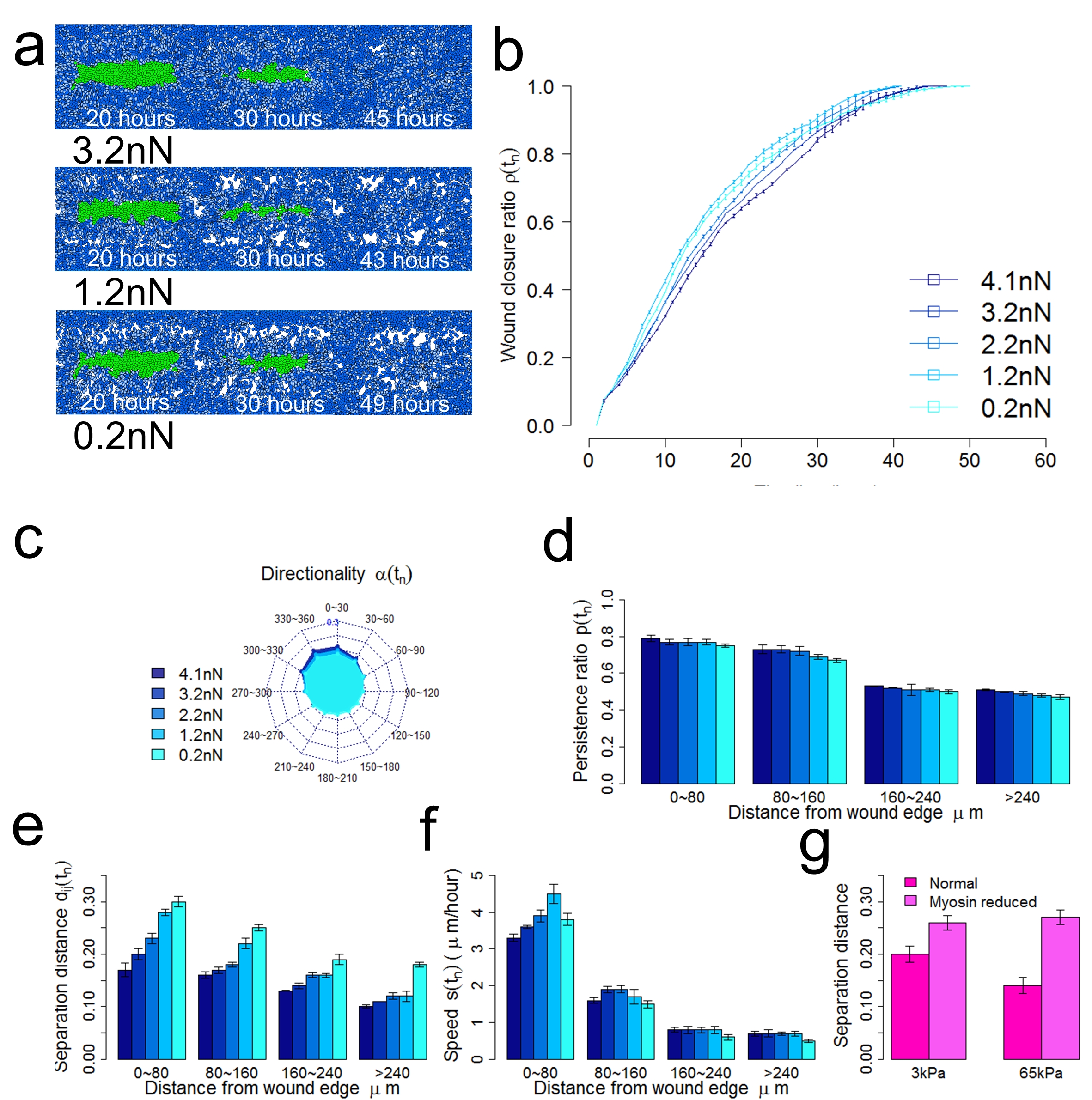
Decreased cell-cell adhesion reduces cell-cell coordination during collective cell migration. (a) Snapshots of tissue at specific time step under decreased cell-cell adhesions. (b) The wound closure ratio over time under decreased cell-cell adhesions. (c) Distribution of direction angle of migrating keratinocytes counted at each time step during re-epithelialization. (d) The persistence ratio of migrating keratinocytes during re-epithelialization. (e) The normalized cell separation distance of migrating keratinocytes during re-epithelialization. (f) Average migration speed of keratinocytes during re-epithelialization. (g) The normalized cell separation distance in study of epithelial cells with reduced myosin-II activity on matrix with different stiffness (Ng, et al., J. Cell Biol., 2012).
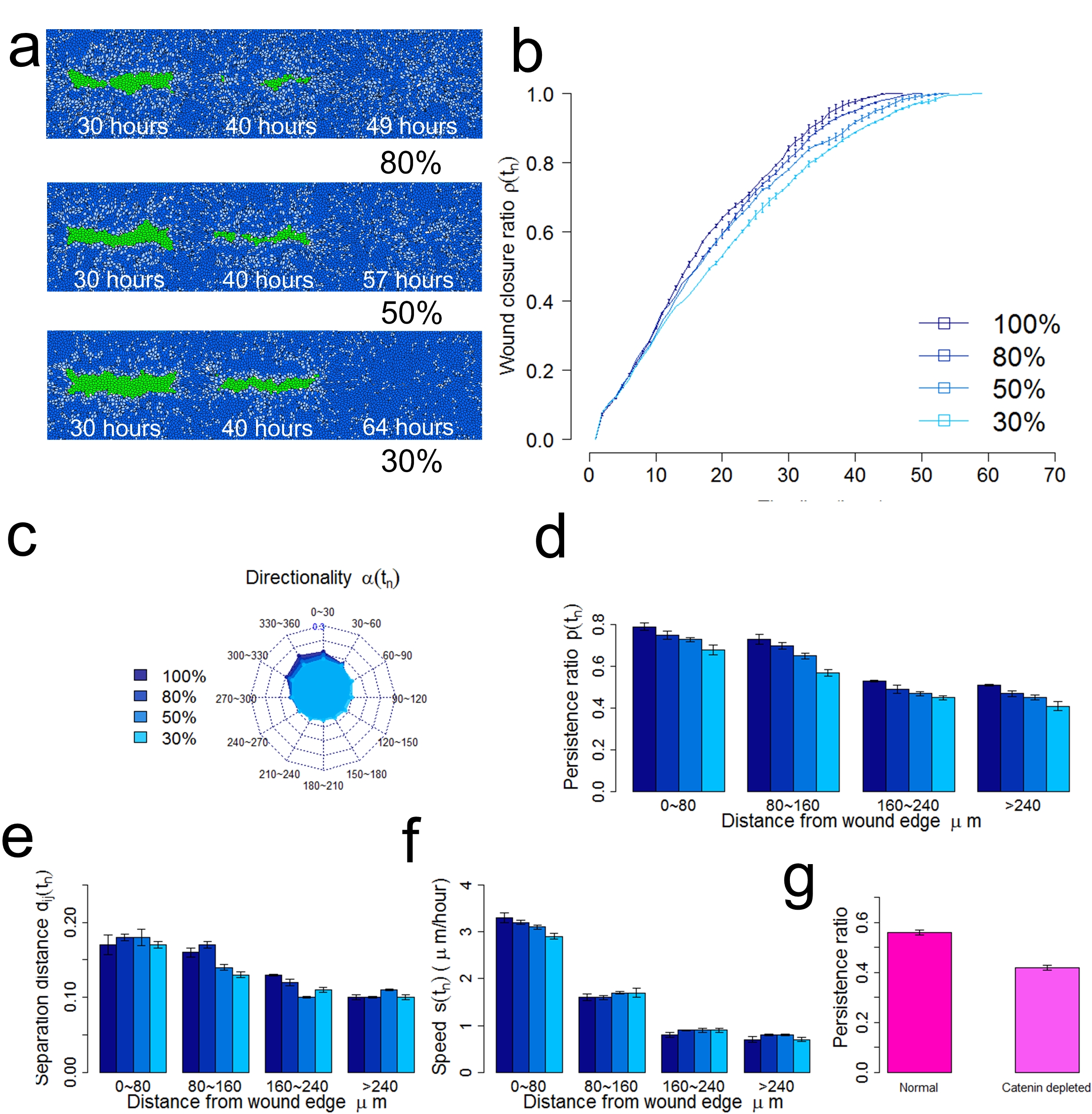
Decreased cell-cell adhesion reduces cell-cell coordination during collective cell migration. (a) Snapshots of tissue at specific time step under inhibited signal transmission. (b) The wound closure ratio over time under inhibited signal transmission. (c) Distribution of direction angle of migrating keratinocytes counted at each time step during re-epithelialization. (d) The persistence ratio of migrating keratinocytes during re-epithelialization. (e) The normalized cell separation distance of migrating keratinocytes during re-epithelialization. (f) Average migration speed of keratinocytes during re-epithelialization. (g) The normalized cell separation distance in study of epithelial cells with depleted αE-catenin level (Benjamin, et al., J. Cell Biol., 2010).
We showed that the decrease of cell-cell adhesion intensity significantly perturbed the cell-cell coordination and reduced the migration directionality. This is in agreement with recent study showing that E-cadherin adhesions and the cortical F-actin cytoskeleton transduce mechanical force to side and rear cells which promotes direction sensing during collective cell migration (Cai, et al., Cell, 2014). We also showed that the inhibition of mechanical force transmission through cell-cell junctions significantly reduced the migration directionality and persistence and prolonged the re-epithelialization time. This characterized more similar with random migration. This suggested that the mechanism to switch from cohesotaxis to chemokinesis (random migration) may be the knockdown or down-regulation of cell-cell adhesion, such as actomyosin or catenin.