Project overview
The behaviors of keratinocytes and fibroblasts are coordinated by cytokines diffusing through the fibrin clot and ECM. Studying the detailed mechanisms of the coordinated cellular processes via traditional laboratory models has been challenging. In this project, we constructed intra-cellular gene regulatory networks stochastically determining the cell behaviors of each individual keratinocyte and fibroblast. We also incorporated intercellular communications via the diffusion of major soluble mediators in the fibrin clot and extracellular matrix. Our results suggests that TGF-β can attenuate the strength of PDGF and KGF signals that fibroblasts and keratinocytes receive by promoting ECM synthesis and deposition in the wound space.
Model setup
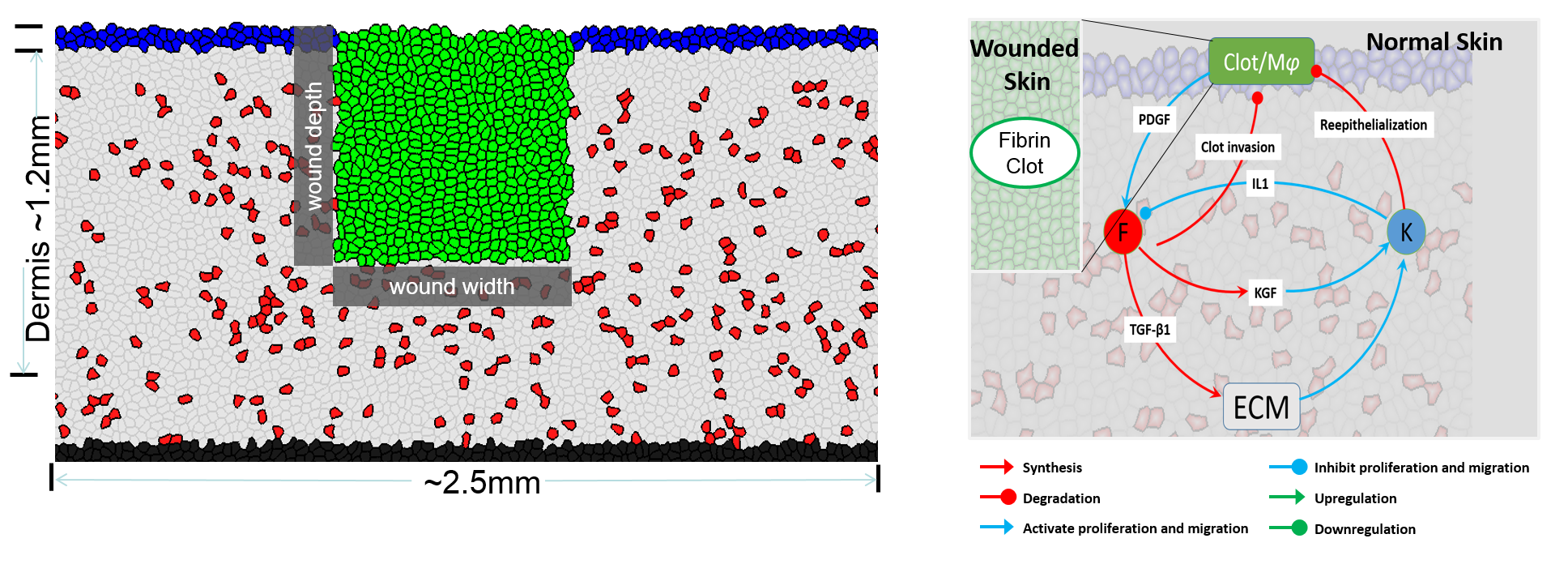
Side view of wound tissue: Green: fibrin clots; Blue: keratinocytes; Red: fibroblasts; Gray: ECM. The cytokine network regulating cell behaviors.
Results
Reproduce the tissue formation process
Our model can reproduce the wound healing process: fibroblasts migrate into the fibrin clots and synthesize new ECM to form the ECM. At the same time, keratinocytes migrate to cover the wound bed.
Re-epithelialization by keratinocytes and ECM reconstruction by fibroblasts at the meantime.
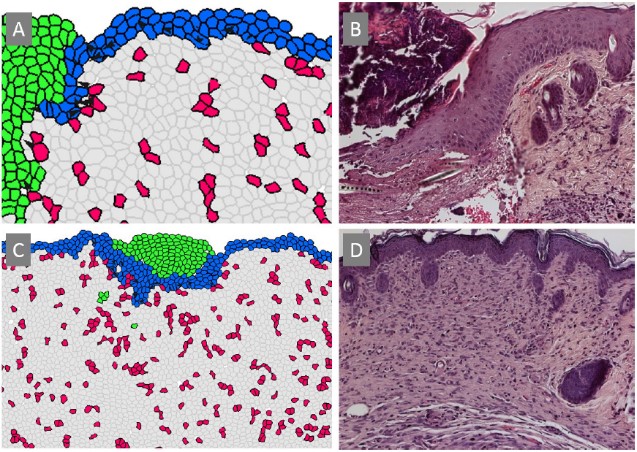
Comparison of tissue patterns between computational predictions (A, C) and experimental results (B, D). The epithelial "migrating tongue" observed in histological imaging in which the epidermal layer invades the wound space and migrates under the fibrin clot to close the wound, is reproduced in the computational simulation.
Effects of TGF-β on wound healing
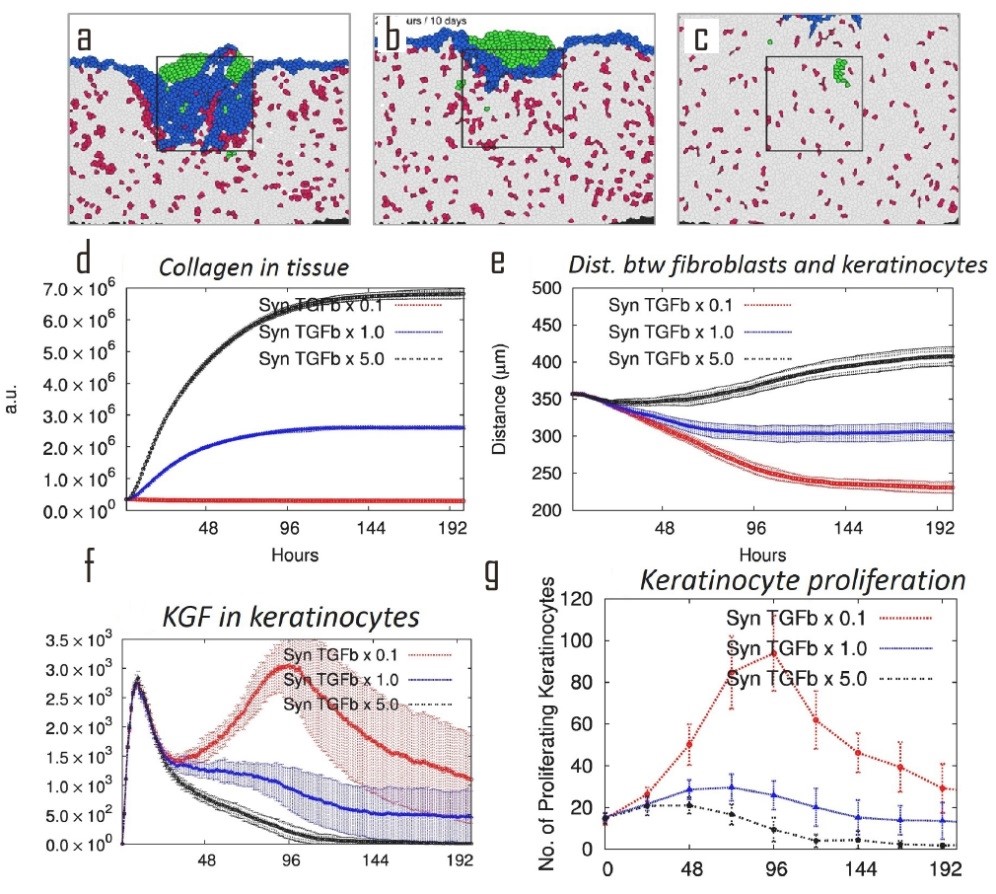
We then perturb the TGF-β by by changing its synthesis rate, and study its overall effects on tissue. The wound tissue pattern was significantly changed under perturbed TGF-β synthesis level. Under reduced TGF-β synthesis level, there were in-adequate ECM generated but hyper-proliferation of keratinocyte in the tissue, which was consistent with the symptoms of the disease hyperplasia (J Chan, et al., J. Exp. Med., 2006). Under increased TGF-β synthesis level, there were over-growth of ECM but inhibited proliferation of keratinocyte in the tissue, which was consistent with the symptoms of the disease hypertrophic scar (A Colwell, et al., Plastic & Recon. Surg., 2005). These similarities suggest perturbation of TGF-β pathway might be the cause of these diseases. Our results suggest that the strength of signals, including PDGF, KGF, and TGF-β, need to diffuse through the wound tissue to reach and act on their target cells. Our results suggest that the strength of signals that cells receive from their local environment can be attenuated by the long-distance transport through the matrix between the signal senders and receivers. When TGF-β is upregulated, collagen production is upregulated, then the collagen density that transmits the signals is increased; the signal can therefore be attenuated due to the vast amount of non-specific interactions between cytokines and ECM components. As a result, cell proliferation is down-regulated. Therefore by regulating the ECM mass, the strength of signals that cells receive can be indirectly regulated; this will eventually influence cell behavior.